Chemistry Form 4 Chapter 7 Acid and Base
The Chapter 1 of Chemistry of Class 11 is Basic concepts of Chemistry. Most commonly an active site amino acid residue is used to accept or donate a proton within the reaction mechanism.
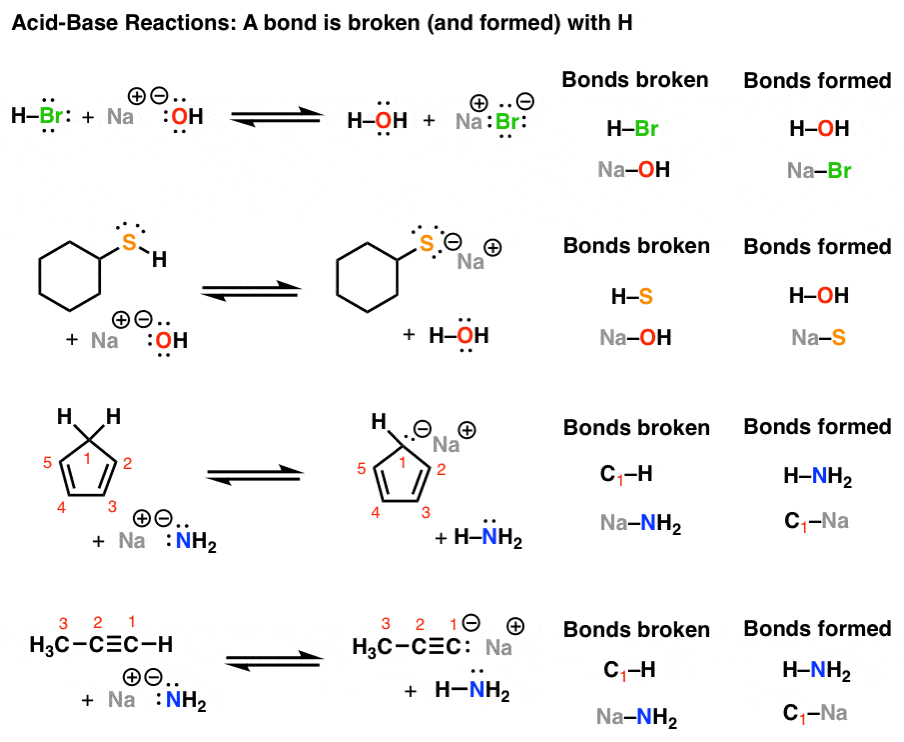
Introduction To Acid Base Reactions Master Organic Chemistry
In general acid-base reactions the pH is usually held.

. This chapter gives an introduction to Chemistry and why it is an important part of science. The pK a value is directly proportional to the standard Gibbs free energy change for the reaction. The acid dissociation constant for an acid is a direct consequence of the underlying thermodynamics of the dissociation reaction.
Solution 8 a Blue litmus will turn. The other sections that could fit within either a general or organicbiological chemistry chapter are sections 56 redox in organic and biochemistry and 75 energy of biochemical reactions. You stop to fill your cars gas tank almost making you late for the first day of chemistry class.
An AP site apurinicapyrimidinic site also known as an abasic site. Acidity is indicated by a pH less than 7 while a pH greater than 7 indicates a base. Figure 127 Abasic Sites.
The value of the pK a changes with temperature and can be understood qualitatively based on Le Châteliers principle. D Change of colour in an acid and a base depends on the type of the indicator. Students can find NCERT Revision notes of Chapter 1 of Chemistry of Class 11 from Vedantu website as well as Vedantu Mobile app.
Important topics covered in NCERT Solutions for class 7 chapter 5 Acids Bases and Salts. PH is a measurement of the proportion of free hydrogen and hydroxyl ions in water. As you find a seat in the classroom you read the.
Get free access to Acids Bases and Salts Class Solutions which includes all the exercises with solved solutions. A base reacts with an acid to form a salt and water only. This type of reaction is known as neutralisation.
The differences listed above depicts the clear difference between acids and bases which forms part of the chemistry and discussed among students the world over. Apurinic and Apyrimidimic AP sites occur due to unstable hydrolysis. Concise Chemistry Part II - Selina Solutions for Class Chemistry ICSE Chapter 3.
The online notes for Chapter 1 of Chemistry of. If section 46 were moved to chapter 12 then 56 and 75 would likely need to be moved into an organic or biological chemistry chapter as well. As we will explore in Chapter 13 methylation of DNA also serves as an important mechanism regulating gene expression.
General acid and general base reactions occur when molecules other than hydronium ion H 3 O or a hydroxide ion OH are the source of proton donation or acceptance. I All four ii a and d iii b c and d iv only d. Which of these statements are correct.
Iv Only d is correct. Another perhaps simpler way to predict the outcome of this reaction is to use the pK a values of the two acids CH 3 CO 2 H 48 and H 2 O 14 clearly acetic acid is a much stronger acid than water and therefore the equilibrium position for this reaction will lie over to the right in favor of the weakest acid and the weakest base.

Acids And Bases Chapter 14 Acid Base Chemistry Acid Base Chemistry Characteristics Acidsbases Ph Less Than 7ph Greater Than 7 Donates H Ionsaccepts Ppt Download
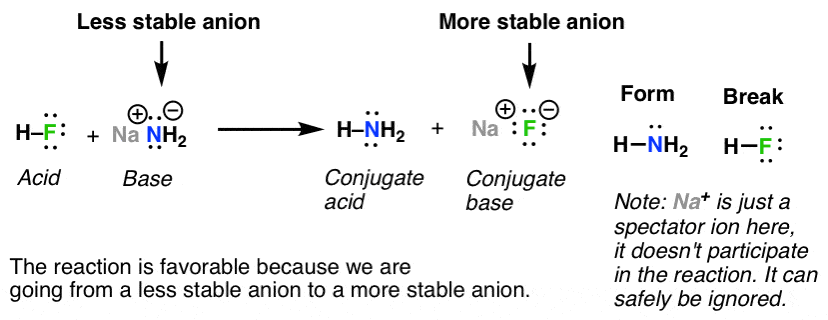
Acid Base Reactions In Organic Chemistry Master Organic Chemistry

4 3 Acid Base Reactions Introduction To Chemistry

Acids And Bases Definition Examples Properties Uses With Videos Faqs
No comments for "Chemistry Form 4 Chapter 7 Acid and Base"
Post a Comment